What Do Pharmaceuticals and the Military Have in Common? The Highest Quality Standards.
Date: August, 2017 | Category: Quality | Author: Ryan Szporer
The pharmaceutical and defense sectors may be at opposite extremes. There is still something to be said in the former for the built-in fail-safes and redundancies that go hand in hand with any product that even hopes to meet military standards.
Military Standard vs. Specification

There’s even an official term for it: MIL-STD for, you guessed it, military standard. At times mistaken for MIL-SPEC, for military specification, MIL-STD is related to the former, but actually something else altogether. Military specifications refer to a given product’s characteristics, so something that meets military specifications is good to go.
Military standards in a sense go deeper, referring to the processes and materials that went into manufacturing that product. One leads to the other.
To illustrate just how large a list of such standards can get, MIL-STD-962D, for one example, covers standards pertaining to well, documenting military standards, for everything ranging from design criteria to manufacturing processes.
You won’t find anything in there about writing Department of Defense handbooks, though. All that’s covered in MIL-STD-967. Seriously. All that to say, that discrepancy in definitions between military standards and specifications is what establishes common ground between defense and pharma. A product manufactured by the pharmaceutical industry can in theory meet military specifications, but it’s how it’s manufactured that is of most interest here.
Reliability Above All Else

You’re ultimately looking for products that are reliable. Reliable translates to “repeatable,” which can only be guaranteed to a mathematically acceptable degree through proper testing. Needless to say, proper testing is one of the pillars of success within the pharmaceutical industry, one in which customers’ health and safety are consistently at stake.
In the interest of full disclosure, it’s not as if pharmaceutical companies absolutely need to follow MIL-STD. GAMP, or Good Automated Manufacturing Practice, already exists, having been founded in 1991. That would be pharma’s own set of guidelines for manufacturers and users of automated systems. GAMP5, released in 2008 by the International Society for Pharmaceutical Engineering, is now in place as the last major revision.
Quality management procedures like Six Sigma helps to further ensure errors and waste are kept to a minimum. That just further proves the point. It becomes abundantly clear how the same principle holds true across the board. There’s an overriding need for a high-quality standard throughout the supply chain. That extends to the packaging that finds its way into the hands of customers, packaging that is an extension, in its own right, of the company’s brand. Any mistakes on a carton at best reflects poorly on a firm. At worst, if there’s a typo that misrepresents the dosage, it could be fatal.
The Highest Quality (Control) Standards
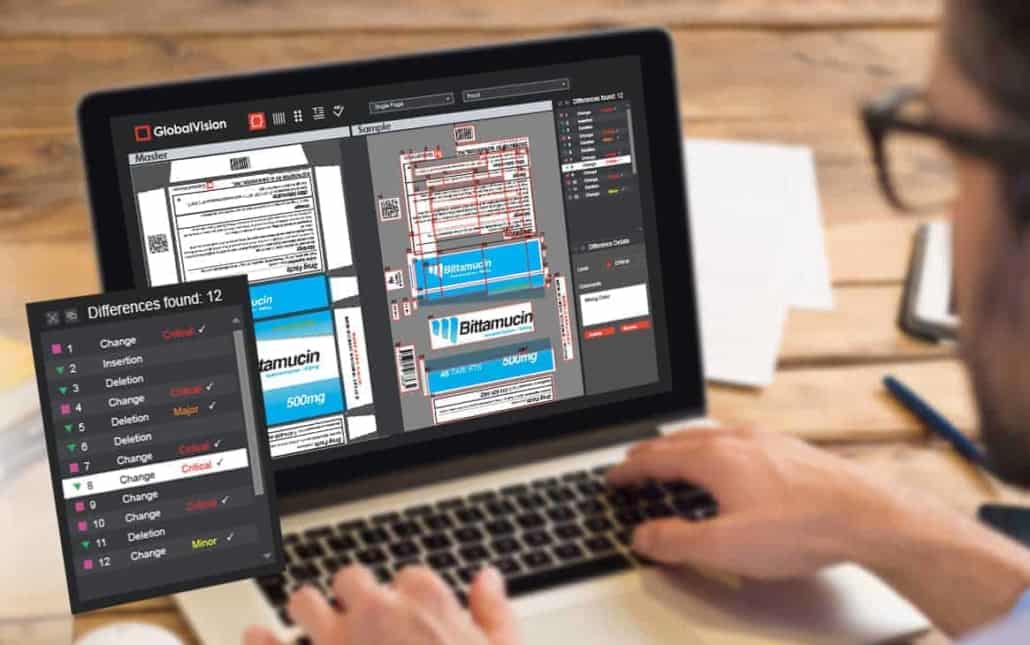
Automated proofing is the best way to protect against errors in packaging. Rest assured, any piece of software on the market has more likely than not undergone more of the same rigorous testing to help guarantee the highest quality standards. After all, that piece of software is ultimately just another product, albeit belonging to a separate industry, but one in which quality is just as paramount.
Extensive system testing and lifecycle documentation covering things like functional requirements prove the software is robust, and that products out of the brand company will be too. This lets managers sign off with confidence on sending a shipment of the packaging off to production when only relatively few samples have been approved.
Before getting more into the sampling process in a future post, it’s important to note why a company would impractically choose not to go through each and every packaging component. On the surface, it may seem like cutting corners just to save money. In reality, it’s devoting the man-hours to areas where they’re better suited because the right system meets quality standards in place much more reliably and consistently than the human eye.
Imagine assigning one or two employees the task of ensuring thousands upon thousands of carton samples are all the same, well, you hope are the same, anyway. High-quality standards take things like hope out of the equation, leaving time and cost savings as happy by-products. The high quality is the real endgame.
GlobalVision is the leading developer of quality control technologies for retail and pharmaceutical packaged goods. Learn how GlobalVision has helped top Pharmaceutical Companies in quality control.
Register for one of our webinars or request a personalized demo today to find out more about how automated quality control can streamline your packaging process.
GET ALL OF OUR LATEST UPDATES
Be the first to receive the latest from GlobalVision.








Military Standard vs. Specification good to read about this. But in pharmaceutical company everything should be followed by BP or USP or ICH or others regulatory guideline.